

Summary
Bronchopleural fistula (BPF) represents a pathological communication between any part of bronchial tree and the pleural space. Mostly it is a rare yet serious complication of different pulmonary conditions like certain infections, trauma, malignancy and/or surgery, going along with significant mortality reported to be as high as 58%. Diagnosis of BPF is mostly done by evaluating these three data: clinical appearance, bronchoscopy and CT scan. The treatment of BPF may initially differ according to the time of appearance as well as clinical presentation. As most BPF goes along with empyema, it is, obviously, necessary to deal with it. This is most commonly achieved with a chest tube. Conservative management of BPF can be achieved using a significant variety of materials and methods with reportedly moderate to excellent results in the treatment of etiologically and sizably different BPF. Methyl-2-cyanoacrylate, fibrin sealant together with spongy calf bone, polidocanol - hydroxypolyethoxidodecane, amplatzer device, silver nitrate, stenting and endobronchial valves have all been employed in bronchoscopic treatment of a BPF. There is no evidence based guideline for its management, either surgically or endoscopically. The treatment of choice is always in the hands of the physician, preferably armed with the current knowledge and capacity of utilizing it.Introduction
BPF represents a pathological communication between any part of bronchial tree and the pleural space. Although it could appear even idiopathic, mostly it is a rare yet serious complication of different pulmonary conditions like certain infections, trauma, malignancy and/or surgery, going along with significant mortality, which is reported to be as high as 58% [1].BPF is thought to be most frequent after surgery [2], having a published overall incidence of 4.4% after major anatomical resections [3], being as high as 20% after pneumonectomy [4]. Predisposing factors for development of BPF may be: immunosuppression, neoadjuvant tumor therapy, presence of pulmonary infection in the time of surgery and active smoking. Also surgical technique may have certain importance on avoiding it [5]. Also it is well known that certain surgical procedures, such as right sided pneumonectomy or right lower lobectomy, go along with increased risk for developing BPF.
Modern radio- and chemotherapy therapies for lung cancer as well as necrotic lung infections may add significantly to overall morbidity from BPF. Furthermore, some other conditions, like ARDS, or even Boerhaave Syndrome could also be responsible for it BPF development.
Surgical BPF can be generally classified according to the time of occurrence as "early" if it occurs in up to 7 days after surgery, "intermediate" if it appears in the time between 8 and 30 days following surgery and, finally, "late" after that period [6].
Diagnosis of BPF is mostly done by evaluating these three data: clinical appearance, bronchoscopy and CT scan. Rarely additional procedures like instillation of methylene blue and its visualisation in the chest tube can be needed.
Treatment
The treatment of BPF may initially differ according to the time of appearance as well as clinical presentation. If it appears acute, especially with life threatening conditions like flooding of the contralateral lung and respiratory insufficiency, the measures must be taken to clear the airway paths and to decompress involved hemithorax with a chest tube.
As most BPF goes along with empyema, it is, obviously, necessary to deal with it. This is most commonly achieved with a chest tube. Moreover it is often required to treat debilitation and multiple comorbidities, which often accompanies BPF.
Accepting that each of the procedures has its own benefits and misfits, surgical repair has had historically bigger role in handling "surgical" BPF, especially with "earlies", up to 7th postoperative day. Different surgical procedures have been involved in dealing with this problem: rethoracotomy with bronchial stump closure and covering with some of the thoracic wall muscles or omentum, sternotomy with the same idea, thoracoplasty either staged or not, fenestration or simple chest tube as a long term therapy. Some of these procedures being rather aggressive are not well tolerated by all BPF patients.
Conservative management of BPF can be achieved using a significant variety of materials and methods that could be employed with reportedly moderate to excellent results in the treatment of etiologically and sizably different BPF.
Bronchoscopic Treatment of Bronchopleural Fistula
The first two reported successful bronchoscopic management of BPF were published back in 1977 by two independent groups, Ratliff et al. [7] and Hartmann et al. [8] respectively. Both of them described their single case of peripheral BPF successfully closed using a sterilized fishing weight to block previously identified (sub)segmental bronchus in the first and using tissue glue, methyl-2-cyanoacrylate, and simply filling up segmental bronchi of a right upper lobe in the second case.
Almost twenty years later, the same material, methyl-2-cyanoacrylate, was successfully employed in closing up of 10 out of 12 central BPF up to 0.5 cm [9]. The glue firms after 10 seconds, so it was relatively easy to keep it in situ. Apart from them, other groups have also successfully treated this problem in a similar way with different success rates and published their experiences as case reports [10-12].
Another landmark study describing a novel technique for closing central BPF dates back in 1998 [13]. Hollaus et al. employed either only fibrin sealant, for the BPF 3 mm or less, or together with spongy calf bone, which was stuck in the fistula minimizing the orifice and preventing dislocation of the fibrin glue into the pleural cavity . The overall rate of successful fistula closure was 35.6%. Fistulas bigger than 8mm were not considered for endoscopic closure.
Varoli et al. in 1998 [14] published their study describing another method in closing central BPF. They successfully treated 23 out of 35 consecutive patients with central postresectional BPF with multiple and repeatedly submucosal injections of polidocanol - hydroxypolyethoxidodecane (Aethoxysklerol Kreussler) on the margins of the fistula. In contrast to other methods, they managed to close fistula up to 10mm diameter.
The next big step in bronchoscopic handling of both central and peripheral BPF was made in 2008 with an article published by Kramer et al. [15]. They used Amplatzer device (AD) to close 5 mm large central postresectional BPF in two of their patients. Amplatzer device has been developed and used mostly either for treatment of congenital heart disorders or, in case of the vascular plug, endovascular catheter embolization of abnormal vascular communication. Both are easily applicable and available in different sizes. ASD-occluder has a central waist from 4-40 mm and disks on both sides of it, which are at least 10mm larger. Amplatzer vascular plug is available in sizes from 4-16 mm, but in at least three different shapes. While the size of the vascular plug is suitable for most working channels of modern fiberoptic bronchoscopes and thus could be easily delivered under optical guidance, ASD-occluder could be challenging. A larger case series was published later on, again by the same group [16]. They first introduced a guide wire through the fistula using bronchoscope. After bronchography to evaluate the shape and length of the fistula, they introduced a delivery sheath over the wire through the defect, than AD on the same fashion, positioning it at the end under bronchoscopic guidance by extruding the central waist in the defect, both of the disks laying on the opposite sides of it. Nine out of ten of the patients in their cohort have been successfully treated with this technique, the only one unsuccessful case was due to a failed anchoring of the device and it"s falling into the pleural cavity. The same group published their results with vascular plug device, having five out five successful treatments [17]. Meanwhile there is a raising published evidence in terms of case reports supporting this method in the treatment of central BPF [18-20]. The largest successfully healed fistula was 12 mm in diameter. Our own experience however showed only limited success, having only two out of four BPF fistula completely closed without involvement of some additional procedure (Figures 1-3).
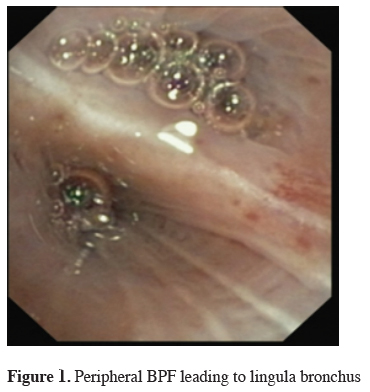 Click Here to Zoom |
Figure 1: Peripheral BPF leading to lingula bronchus |
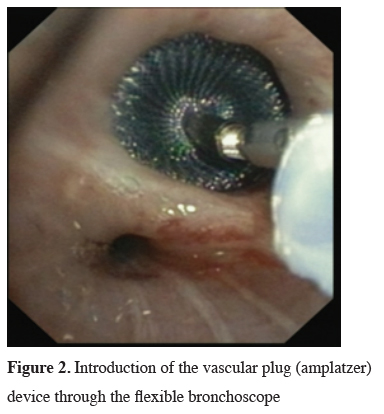 Click Here to Zoom |
Figure 2: Introduction of the vascular plug (amplatzer) device through the flexible bronchoscope |
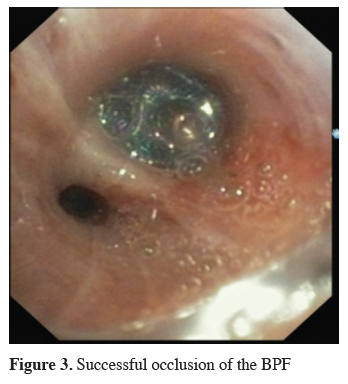 Click Here to Zoom |
Figure 3: Successful occlusion of the BPF |
Silver nitrate has been also employed as a tool for closing central BPF. In 2013 Boudaya et al. [6] published their series consisting of 17 patients treated with local application of silver nitrate. This application has had to be repeated until the fistula was closed. 16 patients was healed, the largest fistula was 9mm in diameter.
Endoscopic stenting as a solution for central BPF was promoted in 2001 by Watanabe et al. [21]. They used Dumon stents to cover central BPF after right upper lobectomy and failed attempt to close it surgically. From that point on there have been numerous case reports during past years with this particular type of stent having the same result, but also with some others, having different results. Koh et al. [22] successfully treated 20 mm large BPF after right upper lobe resection with the Dumon stent. Self-expandable metallic customized stent was employed as a temporary solution in treatment of seven post-pneumonectomy fistulas, the largest having a diameter of 12 mm [23]. The air leak ceased in all patients, in 43% of them surgery was successfully delayed, however overall mortality was 57%. Andreetti et al. [24] published their experience in management of post-pneumonectomy BPF. In their series of six patients having BPF up to 11 mm, but no empyema, they successfully treated all six of them by placing full covered self-expandable nitinol stents. The stents were removed after 71-123 days with completely cured BPF.
Another material frequently being employed in the treatment of BPF is endobronchial valves. The first successful case was published by Snell et al. in 2005 [25]. They closed a large peripheral BPF with a one-way valve, by positioning it in small segmental bronchi that was proved to be connected with the fistula. After that first report, other similar case reports with the same results were published [26,27]. In 2015 two series with valves were published. First study evaluated retrospectively the treatment of 21 patients, 19 of whom had peripheral BPF. None of those 19 patients tolerated water seal drainage system or even the ambulatory pleural drainage system well. 15 of 19 had prolonged air leak one week after treatment and 11 had it longer than two or more weeks. Two patients with central BPF had post-pneumonectomy fistula and were treated with the combination of a stent and a valve, one of them successfully, and the other unsuccessfully, followed by surgery [28]. The second study retrospectively enrolled 14 patients treated for peripheral BPF with overall success in six patients in the "medical group" (50%) and for eight in the "surgical group" (62.5%). Median time for air leak cessation in days for both groups was around 15 days [29].
Conclusion
As a conclusion, bronchopleural fistula is very serious complication of many medical conditions, however most frequently after surgery. There is no evidence based guideline for its management, either surgically or endoscopically.Apart from the surgical management, which is more common given the relative higher frequency of the surgical patients among affected population with BPF, endoscopic management offers some benefits that should not be ignored. Not only it is less invasive, but in some occasions even more efficient than surgery. Avoiding the single anecdotal experience of endoscopic closing of either central or peripheral BPF with plethora of different materials and methods such as: blood clot, gel foam, antibiotics or cellulose without adequate published confirmation from other investigators later on, above mentioned endoscopic strategies have confirmed themselves as valid options in the treatment of BPF. Still not all are equally convenient for all types of BPF.
Central BPF, clearly visualized and measurable could be treated according to its diameter and location. Certainly proximal central BPF, like one after pneumonectomy or right upper lobe resection, with a diameter larger than 10 mm, reduces possibilities to stenting or placing AD. Left sided lobar BPF as well as BPF of the lower lobes on the right, especially if big, may be not amenable for stenting, thus leaving AD as the only method of choice.
Smaller BPF, regardless of the position, may involve other relatively successful tools like silver nitrate or polidocanol - hydroxypolyethoxidodecane, later being challenging, given the fact that, although published as a cohort, it has never been proven by other investigators but only one, unlike silver nitrate, stents or AD.
For the centrally located BPF smaller than 5 mm in diameter, the solid options can be either methyl-2-cyanoacrylate or amplatzer vascular plug and for the smallest fistulas even fibrin glue with or without calf bones.
Peripheral BPF are often treated by existing chest tube either only with suction/no suction combination or installation of certain chemicals or materials. Endoscopic treatment may be efficient as well, in the first place with endobronchial valves. However even this option does not offer a very good efficacy when compared to its risks. Another tool for treatment of peripheral BPF may be amplatzer vascular plug, though once epithelized, it is not removable anymore.
The treatment of choice is always in the hands of the physician, preferably armed with the current knowledge and capacity of utilizing it.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The author received no financial support for the research and/or authorship of this article.
Reference
1) Uramoto H, Hanagiri T. The development of bronchopleural fistula in lung cancer patients after major surgery: 31 years of experience with 19 cases. Anticancer Res 2011; 31: 619-24.
2) Lois M, Noppen M. Bronchopleural fistulas: an overview of the problem with special focus on endoscopic management. Chest 2005;128:3955-65.
3) Sirbu H, Busch T, Aleksic I, Schreiner W, Oster O, Dalichau H. Bronchopleural fistula in the surgery of non-small cell lung cancer: incidence, risk factors, and management. Ann Thorac Cardiovasc Surg 2001; 7: 330-6.
4) Cerfolio RJ. The incidence, etiology, and prevention of postresectional bronchopleural fistula. Semin Thorac Cardiovasc Surg 2001; 13: 3-7.
5) al-Kattan K, Cattelani L, Goldstraw P. Bronchopleural fistula after pneumonectomy for lung cancer. Eur J Cardiothorac Surg 1995; 9: 479-82.
6) Boudaya MS, Smadhi H, Zribi H, Mohamed J, Ammar J, Mestiri T, et al. Conservative management of postoperative bronchopleural fistulas. J Thorac Cardiovasc Surg 2013; 146: 575-9.
7) Ratliff JL, Hill JD, Tucker H, Fallat R. Endobronchial control of bronchopleural fistulae. Chest 1977; 71: 98-9.
8) Hartmann W, Rausch V. New therapeutic application of the fiberoptic bronchoscope. Chest 1977; 71: 237.
9) Scappaticci E, Ardissone F, Ruffini E, et al. Postoperative bronchopleural fistula: endoscopic closure in 12 patients. Ann Thorac Surg 1994; 57: 119-22.
10) Roksvaag H, Skalleberg L, Nordberg C, Solheim K, Hoivik B. Endoscopic closure of bronchial fistula. Thorax 1983;38: 696-7.
11) Torre M, Chiesa G, Ravini M, Vercelloni M, Belloni PA. Endoscopic gluing of bronchopleural fistula. The Ann Thorac Surg 1987; 43:295-97.
12) Wood RE, Lacey SR, Azizkhan RG. Endoscopic management of large, postresection bronchopleural fistulae with methacrylate adhesive (Super Glue). J Pediatr Surg 1992; 27: 201-2.
13) Hollaus PH, Lax F, Janakiev D, Lucciarini P, Katz E, Kreuzer A, et al. Endoscopic treatment of postoperative bronchopleural fistula: experience with 45 cases. Ann Thorac Surg 1998; 66: 923-7.
14) Varoli F, Roviaro G, Grignani F, Vergani C, Maciocco M, Rebuffat C. Endoscopic treatment of bronchopleural fistulas. Ann Thorac Surg 1998; 65: 807-9.
15) Kramer MR, Peled N, Shitrit D, Atar E, Saute M, Shlomi D, et al. Use of Amplatzer device for endobronchial closure of bronchopleural fistulas. Chest 2008; 133: 1481-4.
16) Fruchter O, Kramer MR, Dagan T, Raviv Y, Abdel-Rahman N, Saute M, et al. Endobronchial closure of bronchopleural fistulae using amplatzer devices: our experience and literature review. Chest 2011; 139: 682-7.
17) Fruchter O, Bruckheimer E, Raviv Y, Rosengarten D, Saute M, Kramer MR. Endobronchial closure of bronchopleural fistulas with Amplatzer vascular plug. Eur J Cardiothorac Surg 2012; 41: 46-9.
18) Scordamaglio PR, Tedde ML, Minamoto H, Pedra CA, Jatene FB. Endoscopic treatment of tracheobronchial tree fistulas using atrial septal defect occluders: preliminary results. J Bras Pneumol 2009; 35: 1156-60.
19) Gulkarov I, Paul S, Altorki NK, Lee PC. Use of Amplatzer device for endobronchial closure of bronchopleural fistulas. Interact Cardiovasc Thorac Surg 2009; 9: 901-2.
20) Klotz LV, Gesierich W, Schott-Hildebrand S, Hatz RA, Lindner M. Endobronchial closure of bronchopleural fistula using Amplatzer device. J Thorac Dis 2015; 7: 1478-82.
21) Watanabe S, Shimokawa S, Yotsumoto G, Sakasegawa K. The use of a Dumon stent for the treatment of a bronchopleural fistula. Ann Thorac Surg 2001; 72: 276-8.
22) Koh MS, Ling Hsu AA, Thirugnanam A. Novel management of a large chronic bronchocutaneous fistula after lobectomy. Interact Cardiovasc Thorac Surg 2005; 4: 248-9.
23) Dutau H, Breen DP, Gomez C, Thomas PA, Vergnon JM. The integrated place of tracheobronchial stents in the multidisciplinary management of large post-pneumonectomy fistulas: our experience using a novel customised conical self-expandable metallic stent. Eur J Cardiothorac Surg 2011; 39: 185-9.
24) Andreetti C, D'Andrilli A, Ibrahim M, Ciccone AM, Maurizi G, Mattia A, et al. Effective treatment of post-pneumonectomy bronchopleural fistula by conical fully covered self-expandable stent. Interact Cardiovasc Thorac Surg 2012; 14: 420-3.
25) Snell GI, Holsworth L, Fowler S, Ericksson L, Reed A, Daniels FJ, et al. Occlusion of a broncho-cutaneous fistula with endobronchial one-way valves. Ann Thorac Surg 2005; 80: 1930-2.
26) Toma TP, Kon OM, Oldfield W, Sanefuji R, Griffiths M, Wells F, et al. Reduction of persistent air leak with endoscopic valve implants. Thorax 2007; 62: 830-3.
27) Schweigert M, Kraus D, Ficker JH, Stein HJ. Closure of persisting air leaks in patients with severe pleural empyema--use of endoscopic one-way endobronchial valve. Eur J Cardiothorac Surg 2011; 39: 401-3.



