Summary
Background: The aim of this study was to assess the relationship between Glasgow Prognosis Score (GPS) and survival in patients who underwent pneumonectomy due to pN2 non-small cell lung cancer (NSCLC).Materials and Methods: A total of 45 patients who were determined to have pN2 disease after pneumonectomy between 2007 and 2016 were retrospectively analyzed. The patients were assigned a GPS between 0 and 2 as follows: elevated CRP level (>1.0 mg/dL) and hypoalbuminemia (<35 mg/dL) was classified as GPS 2, elevated CRP but albumin >35 mg/dL was classified as GPS 1, and CRP <1.0 mg/dl and albumin >35 mg/dL were classified as GPS 0.
Results: Of the 45 patients included in the study, 42 (93.3%) were male and 3 (6.7%) were female. Eighteen (40%) of the patients had adenocarcinoma and 27 (60%) had squamous cell carcinoma. Skip pN2 (pN0N2) was detected in 10 patients. Mean follow-up time was 28 months. The 5-year survival rate was 40.2%. The main prognostic factors associated with survival were GPS and adjuvant therapy (p = 0.023, p = 0.001).
Conclusions: In this study, there was no relationship between N1 status and survival in pneumonectomy patients with pN2 NSCLC, whereas GPS score and adjuvant therapy were found to be prognostically significant in terms of survival.
Introduction
Lung cancers are currently the leading cause of cancer-related deaths. Early detection of non-small cell lung cancer (NSCLC) is crucial for the success of surgical treatment [1]. Pathologic N2 (pN2) status is a negative prognostic factor in NSCLC. In the literature, the 5-year survival rate for patients with N2 disease varies from 20 to 35%. The International Association for the Study of Lung Cancer (IASLC) argues that N2 disease must be evaluated together with N1 lymph nodes and that N1 disease also impacts survival [2]. Another important factor affecting cancer prognosis is inflammation [3]. Inflammation-based prognostic scoring systems are used to determine the prognosis of cancer patients. The Glasgow Prognostic Score (GPS) is a scoring system that enables the prediction of tumor behavior and patient prognosis based on albumin and C-reactive protein (CRP) levels [3]. There are few studies in the literature related to GPS and survival outcomes in NSCLC. Previous studies showed that GPS was the most important prognostic factor for survival in adenocarcinoma patients [4]. However, there are no published studies on the relationship between GPS and survival in N2 disease.The aim of the present study was to investigate the factors affecting survival in pneumonectomy patients found to have N2 disease and to evaluate the relationship between GPS and survival in NSCLC.
Methods
The study was approved by the institutional review board (No:20189/1525) and conducted in accordance with the principles of the Declaration of Helsinki. We retrospectively evaluated the records of patients who underwent pneumonectomy in our hospital between June 2007 and December 2016 due to NSCLC and were determined to have pN2 disease. A total of 3370 patients with NSCLC underwent surgery during the study period, 395 of whom had pneumonectomy. Patients who received neoadjuvant therapy and those who were lost to follow-up were excluded. As a result, a total of 45 pN2 (8.8%) patients who underwent pneumonectomy were included in the study. The study flow chart is shown in Figure 1.
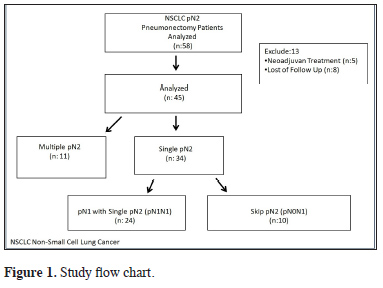 Click Here to Zoom |
Figure 1: Study flow chart. |
Patients found to have single station pN2 were divided into two groups:
i. Patients with single station pN2 skip metastasis (pN0N2)
ii. Patients with single pN1 and single pN2 involvement (pN1N2)
Preoperative Assessment
All patients underwent thoracic computed tomography (CT), positron emission tomography (PET-CT), and cranial magnetic resonance imaging (MRI) during preoperative assessment. Respiratory function tests and electrocardiogram were performed to evaluate pulmonary and cardiac reserve. Carbon monoxide diffusion test and lung perfusion scintigraphy were also done for patients with forced expiratory volume in the first second (FEV1) less than or equal to 40% of the predicted value. Patients with a history of cardiac disease and those aged 60 years or older were evaluated with echocardiogram by a cardiologist. In addition, all patients were examined preoperatively by fiberoptic bronchoscopy. Invasive mediastinal staging was performed by EBUS or mediastinoscopy in all patients with findings of pathological mediastinal lymph node in radiological imaging. The IASLC 2009 lymph node map was used for mediastinal lymph node classification [5].
GPS was determined based on serum CRP and albumin levels before and after surgical treatment. Patients with both high CRP (>1.0 mg/dL) and low albumin (<35 mg/dL) were classified as GPS 2, patients with high CRP but albumin >35 mg/dL were classified as GPS 1, and patients with CRP <1.0 mg/dl and albumin >35 mg/dL were classified as GPS 0.
Postoperative Follow-up
Early mortality was defined as deaths that occurred in hospital or within the first postoperative month. Follow-up information was obtained from all patients through office visits or telephone interviews either with the patient, a relative, or their primary physicians. The patients were followed up by oncologists with thoracic CT and physical examination every 3 months for the first 2 years, every 6 months between 2 and 5 years, and once a year thereafter.
Statistical Analysis
The differences in proportions between groups were compared by using Chi-Square test. Difference between two groups for continuous variables was evaluated by Student’s test. Mann-Whitney U test was used to test the difference between two groups in terms of ordinal or non-normally distributed continuous variables. Survival analysis was conducted using the Kaplan–Meier method and curves were compared using the log-rank test. The level of statistical significance was accepted as p < 0.05 for all analyses.
Results
Of the 45 patients included in the study, 42 (93.3%) were male, 3 (6.7%) were female, and the mean age was 59.4 ± 7.14 years (47-84 years). The patients had a mean smoking history of 35.9 ± 17.8 pack-years. Preoperative CRP and albumin levels were 35.07 ± 44.3 mg/dL and 3.92 ± 0.52 g/dL, respectively. Thirteen patients (28.9%) underwent right-side resection and 32 patients (71.1%) underwent left-side resection. The mean tumor diameter was 5.55 ± 3.21 cm and the mean bronchial resection margin was 2.55 ± 1.98 cm. Eighteen patients (40%) had adenocarcinoma and 27 (60%) had squamous cell carcinoma. Twenty-seven patients (60%) had stage 3A disease and 18 (40%) were stage 3B. The mean number of resected lymph nodes was 19.4 ± 8.26 (8-47). Skip pN2 (pN0N2) was detected in 10 patients. Tumor diameter was significantly larger in patients with GPS 1-2 compared to those with GPS 0 (p = 0.009). There were no statistical differences in GPS according to demographic and histopathological characteristics of the patients (Table 1).Table 1: Comparison of demographic and clinical characteristics of the patients.
Two patients died within the first postoperative month. No statistically significant association was detected between GPS and early mortality (p = 0.509). Eight patients (17.8%) did not receive adjuvant therapy postoperatively due to unstable general condition or refusal of medical treatment.
Mean follow-up time was 28 ± 24.76 months. The 5-year survival rate was 40.2% (median 32 months). The main prognostic factors associated with survival were found to be GPS and adjuvant therapy (p = 0.023, p = 0.001). Prognostic factors associated with survival are shown in Table 2 (Figure 2).
Table 2: Factors associated with survival in pN2 NSCLC patients after pneumonectomy.
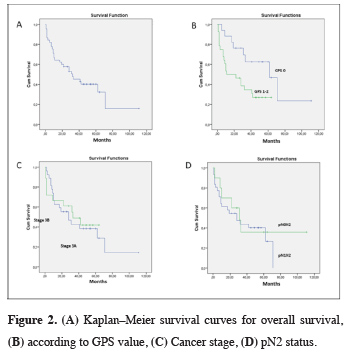 Click Here to Zoom |
Figure 2: (A) Kaplan–Meier survival curves for overall survival, (B) according to GPS value, (C) Cancer stage, (D) pN2 status. |
Discussion
Inflammation in the tumor microenvironment is thought to significantly impact tumor proliferation, angiogenesis, invasion, metastasis, and survival in NSCLC. Systemic inflammatory markers are used to predict prognosis [6]. CRP is a nonspecific inflammatory acute phase protein, and elevated serum CRP levels are observed to adversely affect prognosis [7]. Chronic inflammation may lead to DNA damage, increased cell proliferation, and the development of cancer [8]. CRP is secreted by hepatocytes and is a marker of local inflammation [9]. Albumin is an indicator of nutritional status in cancer patients, and malnutrition has been associated with low survival [10]. In recent years, the GPS has been used to predict cancer behavior and prognosis in patients with colorectal carcinoma in particular. While there are publications before 2015 on the correlation between GPS and pancreatic cancer, hepatocellular carcinoma, trauma, and intensive care unit admission, the number of publications on GPS in NSCLC are limited [4,11,12].In our study, we found that low GPS was among the factors associated with longer survival in pN2 patients who underwent pneumonectomy. The 5-year survival rate was 62.6% for GPS 0 patients versus 27.1% for patients with GPS 1–2 (p = 0.023). In their study on 272 patients with colorectal carcinoma, Nozoe et al. found that survival was significantly worse in GPS 1 patients compared to GPS 0 patients (p = 0.009) and in GPS 2 patients compared to GPS 1 patients (p < 0.0001). Similarly, survival was significantly worse in modified Glasgow Prognostic Score (mGPS) 1 patients compared to mGPS 0 patients (p = 0.009) and in mGPS 2 patients compared to mGPS 1 patients (p = 0.006) [13]. In a meta-analysis by Jin et al. [12] evaluating 5369 NSCLC patients treated with surgery and/or chemotherapy, it was found that elevated CRP levels were associated with shorter survival time (HR = 2.058; 95% CI: 1.51–2.80; p < 0.05). In the same study, survival rate was lower with GPS ≥ 1 compared to GPS 0 (p < 0.001). Minami et al. [14] reported that mGPS was not associated with survival in stage 3B and 4 squamous cell carcinoma patients who received first-line cytotoxic chemotherapy after curative surgery or radiotherapy (p = 0.61).
National Comprehensive Cancer Network (NCCN) guidelines recommend adjuvant chemotherapy for patients determined to have N1 and N2 disease following NSCLC surgery [15]. In their study of patients who received adjuvant therapy after curative resection, Park et al. [16] reported longer disease-free survival after full-dose chemotherapy. In the literature, it can be seen that survival rates are significantly lower among patients who do not receive adjuvant therapy. Speggiari et al. [17] found that the 5-year survival rate in potential N2 patients who received chemotherapy after surgery was 18%, while the survival rate in patients who received chemotherapy alone or chemoradiotherapy alone was 8% (p = 0.02). In our study, postoperative adjuvant therapy was associated with higher survival rate after pneumonectomy in pN2 patients when compared with patients who did not receive adjuvant therapy (p < 0.001).
Skip N2 is observed in 17.2-42.3% of pN2 NSCLC patients [18]. There is still no clear consensus on whether skip N2 has a better prognosis than non-skip N2 [19,20]. This is primarily due to the heterogeneity of pN2 disease. In our study, patients with pN0N2 disease had better 5-year survival than patients with pN1N2. Although these results are similar to those in the literature, there have been some publications indicating otherwise [21]. Legras et al. [22] reported a 5-year survival rate of 25% in patients with N2 disease. Survival was worse in pure N2 patients who were also pN1 (5-year overall survival rate 21% vs. 34%, HR = 2.09, p < 0.001). Similarly, Yazgan et al. [23] also reported much better survival in pure N2 disease compared to pN1N2.
The main limitations of this study are the small patient group and its retrospective design. Furthermore, although it was a single-center study, the surgeries were performed by different surgeons.
In conclusion, based on the findings of this study, N1 status did not affect survival in N2 patients who underwent pneumonectomy due to NSCLC, whereas GPS and adjuvant therapy were significant prognostic factors. Therefore, we believe that assessment with GPS may be beneficial for patients planned to undergo resection in order to determine postoperative prognosis.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research and/or authorship of this article.
Ethics approval
The study was approved by the Ethics Committee of Health of Science University (No: 20189/1525).
Authors’ contributions
CA; Performed the analysis, co-wrote the paper, contributed data/analysis tools, CBS; performed the analysis, co-wrote the paper, AC, MVD; co-wrote the paper, YA, CEK,VE; Collected the data, LC, MM; Contributed data, co-wrote the paper.
Reference
1) Metin M, Citak N, Sayar A, Pekcolaklar A, Melek H, Kök A et al. The role of extended cervical mediastinoscopy in staging of non-small cell lung cancer of the left lung and a comparison with integrated positron emission tomography and computed tomography: does integrated positron emission tomography and computed tomogr. J Thorac Oncol 2011; 6: 1713-9.
2) Rami-Porta R, Goldstraw P. 25 – The Eighth Edition of the Tumor, Node, and Metastasis Classification of Lung Cancer. Second Edi. Elsevier Inc.; 2018. doi:10.1016/B978-0-323-52357-8.00025-1.
3) Al Murri AM, Bartlett JMS, Canney PA, Doughty JC, Wilson C, McMillan DC. Evaluation of an inflammation-based prognostic score (GPS) in patients with metastatic breast cancer. Br J Cancer 2006; 94: 227.
4) Machida Y, Sagawa M, Tanaka M, Motono N, Matsui T, Usuda K et al. Postoperative survival according to the glasgow prognostic score in patients with resected lung adenocarcinoma. Asian Pacific J Cancer Prev 2016; 17: 4677-80.
5) Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009; 4: 568-77.
6) Peebles KA, Lee JM, Mao JT, Hazra S, Reckamp KL, Krysan K et al. Inflammation and lung carcinogenesis: applying findings in prevention and treatment. Expert Rev Anticancer Ther 2007; 7: 1405-21.
7) Jing X, Huang C, Zhou H, Li C, Fan L, Chen J et al. Association between serum C-reactive protein value and prognosis of patients with non-small cell lung cancer: a meta-analysis. Int J Clin Exp Med 2015; 8: 10633.
8) Sayar I, Isik A, Akbas EM, Eken H, Demirtas L. Bone marrow metaplasia in multinodular goiter with primary hyperparathyroidism. Am J Med Sci 2014; 348: 530-1.
9) Morris-Stiff G, Gomez D, Prasad KR. C-reactive protein in liver cancer surgery. Eur J Surg Oncol 2008; 34: 727-9.
10) Alifano M, Mansuet-Lupo A, Lococo F, Roche N, Bobbio A, Canny E et al. Systemic inflammation, nutritional status and tumor immune microenvironment determine outcome of resected non-small cell lung cancer. PLoS One 2014; 9: e106914.
11) Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer 2003; 89: 1028.
12) Jin J, Hu K, Zhou Y, Li W. Prognostic value of the Glasgow prognostic score in lung cancer: evidence from 10 studies. Int J Biol Markers 2018; 33: 201-7.
13) Nozoe T, Matono R, Ijichi H, Ohga T, Ezaki T. Glasgow Prognostic Score (GPS) can be a useful indicator to determine prognosis of patients with colorectal carcinoma. Int Surg 2014; 99: 512-7.
14) Minami S, Ihara S, Komuta K. Pretreatment lymphocyte to monocyte ratio as a prognostic marker for advanced pulmonary squamous cell carcinoma treated with chemotherapy. J Clin Med Res 2018; 10: 657.
15) National Comprehensive Cancer Network. Non-small cell lung cancer (version 6.2018). 2018. Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
16) Park H, Oh D, Ahn YC, Pyo H, Noh JM, Sun J et al. Role of Adjuvant Thoracic Radiation Therapy and Full Dose Chemotherapy in pN2 Non-small Cell Lung Cancer : Elucidation Based on Single Institute Experience 2017; 49: 880-9.
17) Spaggiari L, Casiraghi M, Guarize J, Brambilla D, Petrella F, Maisonneuve P et al. Outcome of Patients With pN2 “Potentially Resectable” Nonsmall Cell Lung Cancer Who Underwent Surgery After Induction Chemotherapy. Semin. Thorac. Cardiovasc. Surg., vol. 28, Elsevier; 2016, p. 593-602.
18) Gorai A, Sakao Y, Kuroda H, Uehara H, Mun M, Ishikawa Y et al. The clinicopathological features associated with skip N2 metastases in patients with clinical stage IA non-small-cell lung cancer. Eur J Cardiothorac Surg 2014; 47: 653-8.
19) Nakagiri T, Sawabata N, Funaki S, Inoue M, Kadota Y, Shintani Y et al. Validation of pN2 sub-classifications in patients with pathological stage IIIA N2 non-small cell lung cancer. Interact Cardiovasc Thorac Surg 2011; 12: 733-8.
20) Sonobe M, Date H, Wada H, Okubo K, Hamakawa H, Teramukai S et al. Prognostic factors after complete resection of pN2 non–small cell lung cancer. J Thorac Cardiovasc Surg 2013; 146: 788-95.
21) Hanagiri T, Takenaka M, Oka S, Shigematsu Y, Nagata Y, Shimokawa H et al. Clinical significance in the number of involved lymph nodes in patients that underwent surgery for pathological stage III-N2 non-small cell lung cancer. J Cardiothorac Surg 2011; 6: 1-8.






