Summary
Background: In this study, we assessed the long-term results of 11 patients that we operated due to primary pulmonary carcinosarcoma in the light of literature.Materials and Methods: We analyzed 11 patients’ data, who underwent lung resection, retrospectively between 2009-2018. The comorbidity score was calculated according to the modified Charlson Comorbidity Index (CCI).
Results: Of the 11 patients in the study, 10 (90.9%) were male, one was female (%9.1), and the median age of the subjects was 59.18 ± 8.86. Nine of the patients (81.8%) underwent lobectomy, two of them (18.1%) underwent pneumonectomy. As a cellular type, 6 of them have squamous cell carcinoma, 5 of them have adenocarcinoma. Furthermore, as a sarcomatous component, we detected 6 of the patients have spindle cell sarcoma, 3 of them have chondrosarcoma, one of them has osteosarcoma. and one patient has angiosarcoma. Nine complications occur postoperatively in 5 patients (45.9%). The fiveyear survival rate was 36.4%. Patients who received adjuvant chemotherapy, the five-year survival rate was 50%, meanwhile, patients who did not receive adjuvant chemotherapy five-year survival rate was not detected (P = 0.006). If the patients have CCI score is two and below their five-year survival rate was 44.4%, meanwhile if the patients have CCI score is two and above their five-year survival rate was not detected (P = 0.018). Recurrence or metastasis was diagnosed in 7 patients (63.6%) postoperatively. The 5-year disease-free survival rate is 12.5%.
Conclusions: Surgical resection is still the most effective treatment modality for these patients. Adjuvant therapy and comorbidity are detected most important factors that affect the survival rate.
Introduction
Primary pulmonary carcinosarcomas (PPCs) are rare malignant tumors of the lung and account for less than 1% of overall lung malignancies [1,2]. Kika et al first described these tumors as sarcomas containing poorly differentiated non-small cell carcinoma [3].Pulmonary carcinosarcomas are commonly seen in middle-aged men and are associated strongly with smoking. The World Health Organization classifies these malignancies under the subgroup of sarcomatoid carcinomas, and they have a poorer prognosis than do non-small cell lung carcinomas [4,5]. Preoperatively, diagnostic and interventional procedures are limited. In non-metastatic diseases, the gold standard for treatment is complete surgical resection, which is also a diagnostic procedure. Few reports have described patients with PPC who underwent surgery, and the studies in which these patients were included were conducted with small samples [6,7]. At this time, PPCs are believed to behave like sarcomas. In this study, we evaluated long-term results from 11 patients who underwent surgery for PPC based on previous reports.
Methods
A patient database including those who underwent lung resection due to carcinosarcoma was created prospectively between January 2009 and December 2018, and this database was evaluated retrospectively in this study. Data from 11 subjects were included. We could not access data on one patient; thus, we excluded that patient from the study. Comorbidity scores were calculated according to the modified Charlson Comorbidity Index (CCI) [8]. The study was approved by the institutional review board (No: 2020/2251) and was conducted in accordance with the principles of the Declaration of Helsinki.
Patient selection
All patients were evaluated preoperatively with thoracic tomography. To detect distant metastasis, PET/CT and cranial magnetic resonance imaging were performed (Figure 1).
 Click Here to Zoom |
Figure 1: (a) Thoracic CT image of a lesion in the right upper lobe, (b) PET/CT image of the lesion in the right upper lobe, showing increased FDG uptake. |
The pulmonary reserve was determined based on pulmonary function tests. When the forced expiratory volume in the first second was ≤ 40%, we ordered a carbon monoxide diffusion test and lung perfusion scintigraphy. For patients aged > 60 years and those with histories of cardiac problems, consultation with cardiologists and echocardiographic evaluation was performed. Before the operation, endobronchial lesions were evaluated using fiberoptic bronchoscopy. The preoperative mediastinal staging was performed according to the European Society of Thoracic Surgeons and American Thoracic Society guidelines [9].
Postoperative follow-up
Data on morbidities occurring during hospitalization, including hemorrhage, persistent air leakage, atrial fibrillation, pneumonia, acute respiratory distress syndrome (ARDS), acute kidney injury (AKI), and septicemia, were collected. Persistent air leakage was diagnosed when leakage lasted ≥ 7 days. Data on mortality occurring during hospitalization, including intraoperative death, and within 3 months following surgery were collected.
Data on patients’ demographic characteristics, morbidity, duration of hospitalization, mortality, histopathological characteristics, and development of relapse, as well as 5-year survival rates, were analyzed. Patient data, including age, comorbidities, tumor histopathology, tumor stage, adjuvant therapy, induction therapy, and survival, were obtained from hospital records and the national survival database. Patient follow-up included thoracic CT and physical examination, performed together with oncologists. Patients were examined every 3 months for the first 2 years, every 6 months in years 2-5, and annually thereafter.
Statistical Analysis
The chi-squared test and Fisher’s exact test were used to examine relationships among patients’ demographic, clinical, descriptive, and categorical data. For continuous variables, we used Student’s t-test, the Mann–Whitney U test, and Kruskal-Wallis analysis. Kaplan-Meir analysis was used to examine patient survival. To identify factors affecting survival, we used the log-rank test. The significance level was set at p < 0.05. All statistical analyses were performed with the SPSS software package (version 22; SPSS Inc., Chicago, IL, USA).
Results
The sample for this study comprised 10 (90.9%) male patients and one (9.1%) female patient. The patients’ median age was 59.18 ± 8.86 years. Nine (81.8%) patients were aged < 65 years and two (18.2%) patients were in the geriatric age group. The average smoking history was 23.54 ± 8.71 pack-years. The most common symptoms in these patients were coughing (54.5%) and dyspnea (36.3%). One (9.1%) patient was asymptomatic. Six patients had tumors on the right lung and five had tumors on the left lung. Six patients were diagnosed preoperatively, with sarcomatoid carcinoma (n = 1 (9.1%)), malignant mesenchymal tumors (n = 3 (27.2%)), and small round cell malignant tumors (n = 2 (18.1%)). Four (36.3%) patients were diagnosed preoperatively based on the detection of endobronchial lesions. All patients underwent thoracotomy with a posterolateral approach. Nine (81.8%) patients underwent lobectomy and two (18.1%) patients underwent pneumonectomy. Patients’ demographic characteristics are summarized in Table 1.Table 1: Demographic characteristics of the patients.
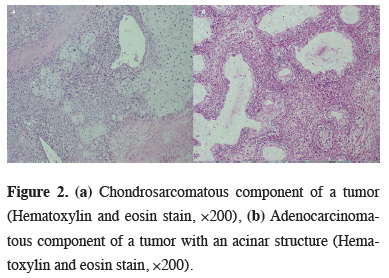
The average tumor diameter was 6.53 ± 5.13 cm (range, 2.20–20 cm). According to tumor size, four (36.4%) cases were designated as stage T1, one (9.1%) as stage T2, two (18.2%) as stage T3, and four (36.4%) as stage T4. Ten of the 11 cases were graded as N0 and one case was graded as N1 during the postoperative period. Five (45.4%) cases were classified as TNM stage IIIA, one (9.1%) as stage IIB, and five (45.4%) as stage I. Histopathologically, six (54.5%) cases were determined to be squamous cell carcinoma (SqCC) and five (45.4%) cases were determined to be adenocarcinoma. Sarcomatous components were spindle cell sarcoma [n = 6 (54.5%)], chondrosarcoma [n = 3, (27.2%)], and osteosarcoma [n = 1 (9.1%)]. One (9.1%) patient had angiosarcoma (Figure 2).
 Click Here to Zoom |
Figure 2: (a) Chondrosarcomatous component of a tumor (Hematoxylin and eosin stain, ×200), (b) Adenocarcinomatous component of a tumor with an acinar structure (Hematoxylin and eosin stain, ×200). |
In total, nine postoperative complications in five (45.9%) patients were recorded. One patient who underwent pneumonectomy presented postoperatively with atrial fibrillation, which could be treated medically. Hemorrhage (400 cc/day) occurred postoperatively in two patients, but did not necessitate revision. Two patients had persistent air leakage; one healed spontaneously and the other was treated with blood patch pleurodesis. One patient developed ARDS after AKI; septicemia occurred and the patient died. Two patients had pneumonia that could be treated medically.
Two deaths occurred within 90 days postoperatively. One patient died because of ARDS, AKI, and sepsis; the other patient died of myocardial infarction while under adjuvant therapy. Postoperative adjuvant therapy was administered to eight patients; one patient died in the early postoperative stage and did not receive such therapy, and two patients had poor general medical conditions precluding it.
The average follow-up period for patients who underwent surgery was 31 months. The median survival duration was 51.1 ± 14.6 months (95% confidence interval (CI), 10.5-51.5 months) and the 5-year survival rate was 36.4% (Figure 3).
 Click Here to Zoom |
Figure 3: Kaplan–Meier curves, (a) overall survival, (b) adjuvant treatment. |
For patients who received adjuvant therapy, the 5-year survival rate was 50%; for those who did not receive adjuvant therapy, the 5-year survival rate was not detected (P = 0.006). The 5-year survival rate for patients with CCI scores ≤ 2 was 44.4%; this rate was not detected for patients with CCI scores > 2 (P = 0.018). Table 2< shows the factors that affected the survival rate. Recurrence or metastasis was detected postoperatively in 7 (63.6%) patients. The average disease-free survival (DFS) period was 18 months (95% CI, 6–29 months) and the 5-year DFS rate was 12%.
Discussion
Pulmonary carcinosarcomas are poorly differentiated biphasic tumors with epithelial and mesenchymal components. These tumors are rare and are considered to form a subgroup of pulmonary sarcomatoid carcinomas [10–12]. PPCs commonly present in men with smoking histories. Huwer et al. [13] reported that 71.4% of patients in their sample were men. According to previous reports, pulmonary carcinosarcoma is four to seven times more common in men than in women [7,10,14,15]. In our sample, 91% of the patients were men and most of them were active smokers.PPCs are more common on average in 60-year-olds and present in three main clinical forms, with endobronchial, peripheric or parenchymatous, or both parenchymal and endobronchial components. Depending on disease location, patients show symptoms such as coughing, dyspnea, hemoptysis, chest pain, fatigue, and loss of weight, or are asymptomatic [16,17]. Tastepe et al. [15] noted that the tumors were located peripherally in 85.7% of cases and that the most common symptom was coughing (57%). Similarly, Sokucu et al. [10] noted that the most common symptom was coughing (66.6%). Koss et al. [18] reported that the tumors were located peripherally in 62% of patients and that symptoms included coughing, dyspnea, and hemoptysis. In our study, 54.5% of tumors had peripheric/parenchymal and endobronchial components and the most common symptoms were coughing (54.5%) and dyspnea (36.3%). One (9.1%) of our patients was asymptomatic.
Preoperatively diagnosed pulmonary carcinosarcoma cases are rare. Davis et al. [19] reported that all of the 17 patients in their sample were diagnosed preoperatively with malignancy, but that only two (11.7%) were diagnosed with carcinosarcoma based on fine-needle aspiration. Similarly, Braham et al. [20] reported that patients were diagnosed preoperatively with non-small cell lung cancer. Devi et al. [17] reported that preoperative biopsies resulted in the diagnosis of poorly differentiated carcinoma. Our findings suggest that the most important factors affecting diagnosis are the biphasic and poorly differentiated nature of the tumors.
Immunohistochemical evaluation of these tumors results in the detection of SqCC as an epithelial component [5,10,15,18,21,22]. Koss et al. [18] noted that epithelial components of these tumors are primarily SqCC (46%) and adenocarcinoma (31%). Tastepe et al. [15] identified SqCC in four (57%) patients and adenocarcinoma in two (28.5%) patients. The most common sarcomatous components are poorly differentiated chondrosarcoma, osteosarcoma, and rhabdomyosarcoma [18,21,23]. Similarly, we detected SqCC as an epithelial component in six (54.5%) patients in our sample. In contrast to previous reports, we detected spindle cell sarcoma in six (54.5%) patients and chondrosarcoma in three (27.2%) patients as sarcomatous components.
Surgical resection is the optimal treatment modality for non-metastatic pulmonary carcinosarcoma. Case series exploring systemic treatment are limited, and the superiority of chemotherapy and radiotherapy to surgery remains controversial [1]. On the other hand, systemic treatment has been successful in part in patients who are not eligible for surgery. Langer et al. [24] reported a period of partial tumor regression and a median survival duration of 9 months in patients with pulmonary carcinosarcoma and chronic obstructive pulmonary disease who received cisplatin + etoposide. However, they detected distant metastasis a few months later. Ersek et al. [1] reported median survival duration of 20 months for patients who underwent surgery alone, 4 months for those who received radiotherapy, and 7 months for those treated with surgery and radiotherapy (p < 0.001). We prefer surgical treatment to systemic treatment for patients with PPC; because of the poorly differentiated and aggressive nature of these tumors, however, the prognosis is poor, with reported survival rates ranging from 20% to 57% [7,10,15,18,25]. Sokucu et al. [10] reported a median survival duration of 9 months. Koss et al. [18] demonstrated that small tumor diameter and early disease stage are good prognostic factors, and reported a 5-year survival rate of 21.3%. Davis et al. [19] reported a 2-year survival rate of 25%, and Xu et al. [25] reported a 2-year survival rate of 43%. In our study, the 5-year survival rate was 36.4%, and the CCI score and adjuvant therapy affected prognosis. Differences in survival rates are due to the examination of small patient populations and differences in the histopathological characteristics of tumor cells (especially in sarcomatous components [13], disease stage, and postoperative treatment modalities.
Postoperative complications occurred in five (45.4%) patients in our sample. The most common complications were persistent air leakage and pneumonia that responded to medical treatment, which occurred in two (18.1%) patients each. These complication rates are higher than reported previously. They were higher in patients with comorbidities, larger tumors, and longer smoking histories. Tastepe et al. [15] reported persistent air leakage in two (28.5%) patients in their sample. Petrov et al. [7] reported minor complications in two patients and bronchopleural fistula (BPF) in one (20%) patient. Sokucu et al. [10] detected BPF in two (33.3%) patients. These reports, however, are case studies with limited numbers of patients.
Postoperative adjuvant therapy improves the prognosis of PPC [10,15,19,25]. Xu et al. [25] reported that chemoradiotherapy improved the 5-year survival rate in five (33.3%) patients. In our study, the 5-year survival rate for patients who received adjuvant therapy was 50% (p = 0.006). We recommend adjuvant therapy during the postoperative period, but further studies of the effects of such therapy are needed.
High pulmonary carcinosarcoma recurrence rates have been reported. Huwer et al. [13] detected local or distant organ metastasis in six (85.7%) patients. Koss et al. [18] reported recurrence in 25 (37.8%) patients, most commonly in the lymph nodes. In contrast, Tastepe et al. [15] reported distant organ recurrence in only one (14.2%) patient. We detected local or distant metastasis or recurrence in seven (63.6%) patients in our sample. The DFS duration was 18 months, and the 5-year DFS rate was 12.5%. As PPCs are biphasic, aggressive, poorly differentiated tumors that behave like sarcomas, the recurrence rate of these tumors is high.
Limitations of this study are its retrospective design and the inclusion of a small number of patients, especially women, may have resulted in bias. Because of the small patient population, a multivariate analysis could not be performed.
In conclusion, as PPCs are rarely diagnosed preoperatively, due primarily to the wide range of variation in their histopathological structure, surgical resection remains the most effective treatment modality. Adjuvant therapy and comorbidities are the most important factors affecting the survival rate. Nevertheless, prognostic factors for these carcinosarcomas have not been identified. Thus, further prospective multicenter studies are needed.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research and/or authorship of this article.
Reference
1) Ersek JL, Symanowski JT, Han Y, Howard A, Dumas K, Ahrens W et al. Pulmonary Carcinosarcoma: A Surveillance, Epidemiology, and End Results (SEER) Analysis. Clin Lung Cancer 2020;21:160–70.
2) Dogru MV, Sezen CB, Aker C, Girgin O, Kilimci U, Erduhan S et al. Evaluation of Factors Affecting Morbidity and Mortality in Pneumonectomy Patients. Acta Chir Belg 2020:1-16.
3) Kakos GS, Williams Jr TE, Assor D, Vasko JS. Pulmonary carcinosarcoma: etiologic, therapeutic, and prognostic considerations. J Thorac Cardiovasc Surg 1971;61:777-83.
4) Oh S, Miyamoto H, Yamazaki A, Fukai R, Shiomi K, Sonobe S et al. Prospective analysis of depression and psychological distress before and after surgical resection of lung cancer. Gen Thorac Cardiovasc Surg 2007;55:119-24.
5) Sezen CB, Kocaturk CI, Bilen S, Kalafat CE, Cansever L, Dincer SI et al. Long-Term Outcomes of Carinal Sleeve Resection in Non-Small Cell Lung Cancer. Thorac Cardiovasc Surg 2020;68:190-8.
6) Bacha EA, Wright CD, Grillo HC, Wain JC, Moncure A, Keel SB et al. Surgical treatment of primary pulmonary sarcomas. Eur J Cardio-Thoracic Surg 1999;15:456-60.
7) Petrov DB, Vlassov VI, Kalaydjiev GT, Plochev MA, Obretenov ED, Stanoev VI et al. Primary pulmonary sarcomas and carcinosarcomas - Postoperative results and comparative survival analysis. Eur J Cardiothorac Surg 2003;23:461-6.
8) Beddhu S, Bruns FJ, Saul M, Seddon P, Zeidel ML. A Simple Comorbidity Scale Predicts Clinical Outcomes and Costs in Dialysis Patients. Am J Med 2000;108:609-13.
9) Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
10) Sökücü SN, Kocatürk C, Ürer N, Sönmezoǧlu Y, Dalar L, Karasulu L et al. Evaluation of six patients with pulmonary carcinosarcoma with a literature review. Sci World J 2012;2012. https://doi.org/10.1100/2012/167317.
11) Ravi V, Antonoff M, Wingate H, Wang W, Hunt KK. Primary Sarcomas of the Lung. Textb Uncommon Cancer 2017:248-65.
12) Sezen CB, Gulgosteren M, Memis L, Tastepe AI. A rare case of primary synovial sarcoma localized in the thoracic inlet. Curr Thorac Surg 2019;4:99-102.
13) Huwer H, Kalweit G, Straub U, Feindt P, Volkmer I, Gams E. Pulmonary carcinosarcoma: Diagnostic problems and determinants of the prognosis. Eur J Cardiothorac Surg 1996;10:403-7.
14) Rossi G, Cavazza A, Sturm N, Migaldi M, Facciolongo N, Longo L et al. Pulmonary carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements: A clinicopathologic and immunohistochemical study of 75 cases. Am J Surg Pathol 2003;27:311-24.
15) Taştepe İ, Gülhan E, Ege T, Demirağ F, Kaya S, Altınok T. Pulmonary carcinosarcomas: an evaluation of seven patients. Turkish J Thorac Cardiovasc Surg 2009;17:36-9.
16) Jenkins BJ. Carcinosarcoma of the lung: Report of a case and review of the literature. J Thorac Cardiovasc Surg 1968;55:657-62.
17) Devi P, Singh N, Tortora MJ. Pulmonary Carcinosarcoma: A Case Report of Biphasic Lung Tumor. Cureus 2019;11:e5643.
18) Koss MN, Hochholzer L, Frommelt RA. Carcinosarcomas of the lung: a clinicopathologic study of 66 patients. Am J Surg Pathol 1999;23:1514.
19) Davis MP, Eagen RT, Weiland LH, Pairolero PC. Carcinosarcoma of the Lung: Mayo Clinic Experience and Response to Chemotherapy. Mayo Clin Proc 1984;59:598-603.
20) Braham E, Rejeb H Ben, Aouadi S, Kilani T, El Mezni F. Pulmonary carcinosarcoma with heterologous component: Report of two cases with literature review. Ann Transl Med 2014;2:2-5.
21) Takeda SI, Nanjo S, Nakamoto K, Imachi T, Yamamoto S. Carcinosarcoma of the lung: Report of a case and review of the literature. Respiration 1994;61:113-6.
22) Kim KI, Flint JD, Müller NL. Pulmonary carcinosarcoma: radiologic and pathologic findings in three patients. AJR Am J Roentgenol 1997;169:691-4.
23) Nakajima M, Kasai T, Hashimoto H, Iwata Y, Manabe H. Sarcomatoid carcinoma of the lung: a clinicopathologic study of 37 cases. Cancer 1999;86:608-16.