

2Department of Pathology, Bolu Abant Izzet Baysal University, Bolu, Turkey DOI : 10.26663/cts.2021.0020
Summary
Background: Pulmonary contusion is a potentially life-threatening clinical manifestation frequently seen in acute thoracic trauma. It can be life-threatening if diffuse in nature. This study investigated the tissue damage-reducing effect of carvacrol, an antioxidant, in rats with induced lung damage.Materials and Methods: Twenty-one female Wistar Albino rats weighing 240-320 g were used in the study. Pulmonary contusion was induced with the application of 2.25 joules of energy with a modification of the isolated bilateral pulmonary contusion model. Lung tissues of rats administered two doses of oral 50 mg/kg carvacrol were then subjected to histopathologic examination.
Results: Congestion, alveolar destruction, alveolar hemorrhage, and leukocyte infiltration were subjected to histopathologic examination in all lung sections. The amount of alveolar hemorrhage was significantly lower in the carvacrol group compared with the control and trauma groups (p = 0.033). Congestion, leukocyte infiltration, and destruction also decreased, albeit statistically insignificantly, in the carvacrol group.
Conclusion: The antioxidant carvacrol significantly prevented alveolar hemorrhage in pulmonary contusion developing following trauma. Congestion decreased, and no leukocyte infiltration developed in the carvacrol group. However, it did not affect destruction.
Introduction
Pulmonary parenchyma damage can develop as a result of blunt or penetrating trauma. Pulmonary contusion is frequently seen following trauma and is responsible for 20-25% of post-traumatic adult deaths [1]. Pulmonary contusion is an independent risk factor for post-traumatic pneumonia, acute lung injury (ALI), acute respiratory distress syndrome (ARDS), ventilator-associated pneumonia, and respiratory distress [2]. Blood extravasation is linked to alveolar damage and the subsequent activation of a series of proinflammatory cells that occur following trauma. An increased blood leukocyte ratio and subsequent increase in tissue macrophage density initiate inflammatory processes, such as cytokine, proteolytic enzyme, and coagulation cascades. This process increases alveolar-capillary permeability, leading to edema, ventilation/perfusion mismatch, and loss of pulmonary compliance [3].Edema and inflammation in the lungs in uncomplicated cases resolve in 48-72h in radiologic examinations. Apart from support therapies, there are no definite therapeutic methods for reducing complications in pulmonary contusion. Free oxygen radicals forming in tissue following trauma can further exacerbate tissue damage. Several previous studies have shown that antioxidant substances reduce complications in pulmonary contusion and exhibit positive effects on tissue healing. Carvacrol is a monoterpenoid phenolic compound (5-isopropyl-2-methylphenol) found in several essential oils. Studies are showing the antioxidant, antitumor, and antiinflammatory effects of calcavrol [4]. One meta-analysis of the in vitro effect of carvacrol identified antimicrobial, bactericidal, antifungal, anti-inflammatory, antioxidant, antidepressant, immunologic modulatory, and mutagenic activities [4].
Pulmonary contusion is a clinical manifestation frequently seen in acute thoracic trauma. This study investigated the tissue damage-reducing effect of carvacrol, an antioxidant substance, in rats with induced lung damage.
Methods
The research was conducted at the Bolu Abant Izzet Baysal University Experimental Animals Center Laboratory using 21 female Wistar Albino rats weighing 240-320 g. All rats were cared for in line with the Care and Use of Laboratory Animals Guideline published by the National Institutes of Health. Approval for the study was granted by the Bolu Abant Izzet Baysal University Medical Faculty Experimental Animals Ethical Committee, Turkey (2020/22).Three groups were established: group 1, the control group, in which no contusion model was established. At the end of the experiment, the rats were sacrificed under general anesthesia, and their lung tissues were sent for histopathologic examination. Group 2: the contusion group, in which a contusion model was established, but in which no drug was administered. The rats were sacrificed under general anesthesia after 48 h, and their lung tissues were sent for histopathologic examination. Group 3: the contusion + carvacrol group. The first dose of carvacrol (50 mg/kg) was administered via the oral route 30 min after induction of contusion. A second dose was administered after 24 h. The rats were sacrificed under general anesthesia after 48 h, and their lung tissues were sent for histopathologic examination.
The doses of carvacrol (cas: 499-75-2) administered in the studies were in the range of 100-500 mg/kg [5,6]. In an animal study, carvacrol was shown to have a half-life of 1.84 to 2.05 hours in the digestive system. The same study also showed that unencapsulated carvacrol was almost completely absorbed in the stomach and proximal small intestine. Plasma levels peaked at 1.39 hours. In rats, the dose produced a toxic dose of 810 mg/kg when administered orally and 80 and 73 mg/kg when injected intravenously or intraperitoneally [6,7].
The trauma model
A bilateral pulmonary contusion model was used based on a modification of the isolated bilateral pulmonary contusion model described by Raghavendran et al [8]. Pulmonary contusion was induced by dropping a 230 g weight onto the thoracic anterior wall from a distance of one meter (Figure 1). The resulting energy was calculated at 2.25 joules using the formula E = mgh (where E = energy, g = gravity, h = height – 100 cm, and the weight dropped = 0.23 kg). Pulmonary contusion was created by applying 2.25 joules of energy to the rats (Figure 1).
 Click Here to Zoom |
Figure 1: The induced trauma model. |
Anesthesia and the Surgical Procedure
Prior to the experimental procedure, rats received general anesthesia using ketamine HCI 40 mg/kg (Ketalar 500 mg flk; Zentiva Health Products San. Tic. A.Ş. Lüleburgaz- Kırklareli) and xylazine 5 mg/kg (Xylased 100 mg/mL; Bioveta medical Çankaya/Ankara/Turkey) via the intramuscular route. Rats continued to breathe spontaneously throughout the procedure. Rats developing no additional complications after the trauma were taken for surgery after 48 h. Sternotomy was performed through an incision over the sternum. Intracardiac access was provided with an injector and blood was drained. Both lung tissues were then extracted by severing the points of attachment to the mediastinum. The lung tissue specimens were placed into formaldehyde solution and sent to the pathology department for histopathologic examination.
Histopathological Examination
Tissues sent for histopathologic examination were divided into five sections. In line with standard procedures, paraffin-embedded tissues were cut into 5-μm sections. These were gradually deparaffinized and hydrated before staining with hematoxylin and eosin. Alveolar congestion, alveolar destruction, alveolar hemorrhage, and leukocyte infiltration in the vascular wall and alveolar space were evaluated in all lung tissue sections in the histopathologic examination. All these parameters were scored semi-quantitatively as none (0), mild (< 10%), moderate (10-45%), or severe (> 45%) (18) (Figure 2).
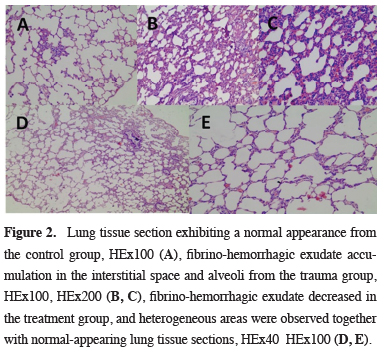 Click Here to Zoom |
Figure 2: Lung tissue section exhibiting a normal appearance from the control group, HEx100 (A), fibrino-hemorrhagic exudate accumulation in the interstitial space and alveoli from the trauma group, HEx100, HEx200 (B, C), fibrino-hemorrhagic exudate decreased in the treatment group, and heterogeneous areas were observed together with normal-appearing lung tissue sections, HEx40 HEx100 (D, E). |
Statistical Analysis
Statistical analyses were performed using the SPSS software. The Kruskal-Wallis test was used to compare continuous data between the groups, and the Chi-square test for non-continuous data. P values of < 0.05 were regarded as statistically significant.
Results
Congestion, alveolar destruction, alveolar hemorrhage, and leukocyte infiltration were evaluated in each lung section in the histopathologic examination. The amount of alveolar hemorrhage was significantly lower in the carvacrol group compared with the control and trauma groups (p = 0.033). Moderate/severe hemorrhage was observed in four (57.1%) rats from the control group and in one (14.2%) from the carvacrol group.Moderate/severe congestion was observed in four (57.1%) rats from the trauma group and one (14.2%) from the carvacrol group. Although the difference was not statistically significant (p = 0.061), noticeably less congestion was determined in the carvacrol group.
Moderate/severe destruction was observed in two (28.5%) rats from the trauma group and one (14.2%) from the carvacrol group. No statistically significant difference was determined (p = 0.31).
The histopathologic examination revealed moderate/intense leukocyte infiltration in two (28.5%) rats from the trauma group. Mild leukocyte infiltration was observed in rats from the carvacrol group. No statistically significant difference was observed (p = 0.11) (Table 1).
Table 1: The study groups’ histopathological data.
Discussion
The results of this study showed a statistically significant decrease in hemorrhage caused by trauma-associated alveolar damage. Leukocyte infiltration, congestion, and destruction also decreased in damaged tissue, although not significantly. Based on these findings, we think that carvacrol exhibits a parenchyma-protecting effect in rats with induced pulmonary contusion. However, further experimental, laboratory, and clinical studies are now needed to confirm this.Blunt chest wall trauma causes diffuse or segmental pulmonary contusion. Local and systemic inflammatory processes are initiated in association with tissue damage, leading to morbidity and mortality [9]. Approximately 25% of trauma-related deaths are caused by chest trauma [10]. Fluid accumulates in the alveolar or interstitial spaces in trauma-associated pulmonary contusion. Ventilation disorder, hypoxia, and hypercarbia develop in the alveoli as a result [9,10].
Free oxygen radicals emerge in association with tissue damage in cases of contusion in the lung. Free radicals are short-lived, unstable, low-molecular-weight, and highly active molecules with one or more unpaired electrons. Free radical production under most circumstances is a part of the pathomechanism, and several xenobiotic toxicities are associated with free radical production. The most important free radicals in the human body are oxygen-derived radicals. Oxygen is reduced to the superoxide group (O2) through the effect of various iron-sulfur-containing oxidation-reduction enzymes and flavoproteins. The superoxide group is highly potent and leads to cell damage and is converted into hydrogen peroxide (H2O2) and oxygen by the copper enzyme superoxide dismutase [11,12]. Antioxidants are substances that directly and indirectly protect the cells against the undesirable effects of xenobiotics, carcinogens, and toxic radical reactions. This study investigated the tissue damage-preventing effect of the powerful antioxidant carvacrol.
In pulmonary contusion caused by blunt thoracic trauma, blood and fluid leak into the alveolar area and interstitial space due to oxidative stress, resulting in interstitial bleeding and edema within a few hours post-trauma [13]. Antioxidant agents reduce parenchymal changes characterized by leukocyte infiltration, excessive bleeding, alveolar impairment, congestion, and alveolar edema [13]. In our study, the decrease in fibrinohemorrhagic exudate accumulated in the alveolar area in the trauma group shows that the anti-inflammatory effect of carvacrol may be strong.
One study involving animal models reported that carvacrol contributed to the healing of acute pancreatitis through antioxidant mechanisms [14]. Recent research involving rats showed that carvacrol treatment healed oxidative stress injury in the brain, liver, and kidneys [15]. A randomized, double-blinded phase II clinical study from 2018 revealed that carvacrol possessed the ability to reduce inflammatory activity and symptoms in patients with asthma [16].
A meta-analysis of 31 publications investigating the in vitro effectiveness of carvacrol published in 2018 also revealed antimicrobial, bactericidal, antifungal, anti-inflammatory, antioxidant, antidepressant, immunomodulatory, and mutagenic activities [17].
Due to its antioxidant properties, carvacrol has also been shown to reduce DNA injury, prevent lipid peroxidation, and provide NO elimination [18]. In addition, its anti-inflammatory effect has also been proved to result from COX-2 suppression [19]. Significantly less hemorrhage was observed in rats administered carvacrol in the present study, and decreases were also observed in congestion and leukocyte infiltration, although these were not statistically significant.
In conclusion, hemorrhage resulting from trauma-related alveolar damage decreased significantly in rats receiving carvacrol in the present study. We also observed decreases in leukocyte infiltration and congestion in damaged tissue from rats given carvacrol, although these were not statistically significant. Based on these findings, we think that carvacrol protects the parenchyma in rats with induced pulmonary contusion, but further experimental, laboratory and clinical studies are needed to obtain more meaningful results.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research and/or authorship of this article.
Ethics Approval
The study was approved by the Bolu Abant Izzet Baysal University Medical Faculty Experimental Animals Ethical Committee, Turkey (2020/22).
Authors’ contributions
OY; conceived of the presented idea and collected the data, SPÖ: developed the analysis. OY, SPÖ; verified the analytical methods, discussed the results and contributed to the final manuscript and co-wrote the paper.
Reference
1) Kao RL, Huang W, Martin CM, Rui T. The effect of aerosolized indomethacin on lung inflammation and injury in a rat model of blunt chest trauma. Can J Surg 2018; 61: S208-S218.
2) Sasser SM, Sattin RW, Hunt RC, Krohmer J. Blast lung injury. Prehosp Emerg Care 2006; 10: 165-72.
3) Türüt H, Ciralik H, Kilinc M, Ozbag D, Imrek SS. Effects of early administration of dexamethasone, N-acetylcysteine and aprotinin on inflammatory and oxidant–antioxidant status after lung contusion in rats. Injury 2009; 40: 521-7.
4) Feng X, Jia A. Protective effect of carvacrol on acute lung injury induced by lipopolysaccharide in mice. Inflammation. 2014 ; 37: 1091-101.
5) Guan X, Li X, Yang X, Yan J, Shi P, Ba L et al. The neuroprotective effects of carvacrol on ischemia/reperfusion-induced hippocampal neuronal impairment by ferroptosis mitigation. Life Sci. 2019; 235: 116795.
6) Michiels J, Missotten J, Van Hoorick A, Ovyn A, Fremaut D, De Smet S et al. Effects of dose and formulation of carvacrol and thymol on bacteria and some functional traits of the gut in piglets after weaning. Arch Anim Nutr 2010; 64: 136-54.
7) Suntres ZE, Coccimiglio J, Alipour M. The bioactivity and toxicological actions of carvacrol. Crit Rev Food Sci Nutr 2015; 55: 304-18.
8) Raghavendran K, Davidson BA, Helinski JD, Marschke CJ, Manderscheid P, Woytash JA et al. A rat model for isolated bilateral lung contusion from blunt chest trauma. Anesth Analg 2005; 101: 1482-9.
9) Miller PR., Croce MA, Kilgo PD, Scott J. Acute respiratory distress syndrome in blunt trauma: Identification of independent risk factors/discussion. Am Surg 2002; 68: 845.
10) Gündoğdu AG, Çamaş HE, Yazkan R. Blunt thoracıc trauma. Med J SDU 2018; 25: 86-97.
11) Janciauskiene S. The beneficial effects of antioxidants in health and diseases. Chronic Obstr Pulm Dis 2020; 7: 182-202.
12) Matés JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 2000; 153: 83-104.
13) Gavelli G, Canini R, Bertaccini P, Battista G, Bnà C, Fattori R. Traumatic injuries: imaging of thoracic injuries. Eur Radiol 2002; 12: 1273-94.
14) Bakır M, Geyikoglu F, Colak S, Turkez H, Bakır TO, Hosseinigouzdagani M. The carvacrol ameliorates acute pancreatitis‐ induced liver injury via antioxidant response. Cytotechnology 2016; 68: 1131-46.
15) Samarghandian S, Farkhondeh T, Samini F, Borji A. Protective Effects of Carvacrol against Oxidative Stress Induced by Chronic Stress in Rat’s Brain, Liver, and Kidney. Biochem Res Int 2016, doi: 10.1155/2016/2645237.
16) Alavinezhad A, Khazdair MR, Boskabady MH. Possible therapeutic effect of carvacrol on asthmatic patients: A randomized, double blind, placebo-controlled, Phase II clinical trial. Phytother Res 2018; 32: 151-9.
17) Silva ER, de Carvalho FO, Teixeira LGB, Santos NGL, Felipe FA, Santana HSR et al. Pharmacological Effects of Carvacrol in In vitro Studies: A Review. Curr Pharm Des 2018; 24: 3454-65.



